FDA Approves Glossy Option for Rosacea
— Antibiotic Emrosi topped doxycycline, placebo in allotment III trials
by Ian Ingram, Managing Editor, MedPage This day November 4, 2024
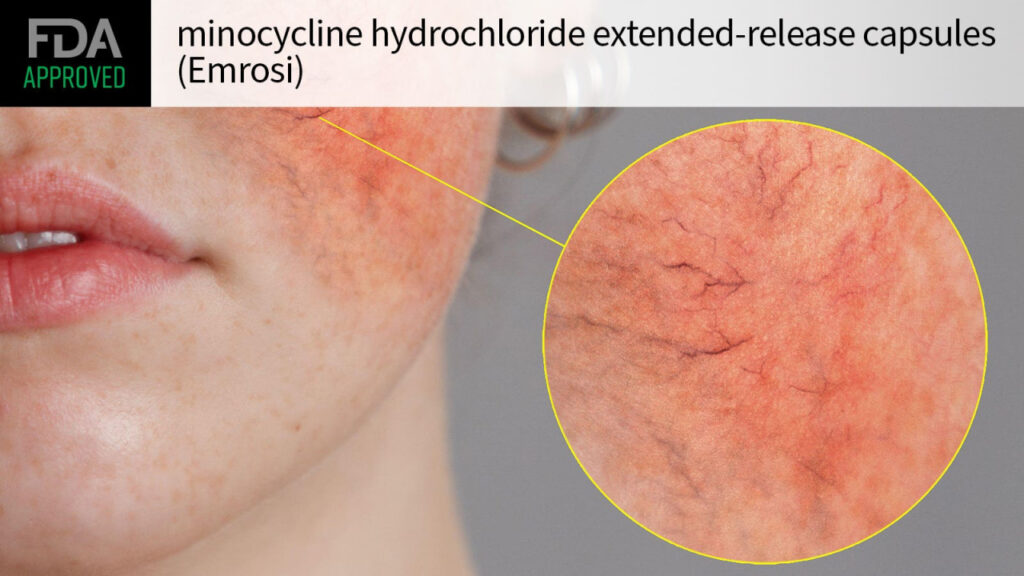
The FDA permitted minocycline hydrochloride prolonged-release capsules (Emrosi) for treating inflammatory lesions of rosacea in adults, drugmaker High-tail Scientific announced on Monday.
Approval of the tetracycline antibiotic for this indication become as soon as supported by records from two multicenter allotment III trials — MVOR-1 and MVOR-2 — entertaining 653 adults with papulopustular rosacea.
Contributors had been randomized to both day-after-day minocycline hydrochloride (40 mg); doxycycline, the present current of care; or placebo for 16 weeks. At baseline, people had not not up to 15 papules or pustules (suggest 25 inflammatory lesions) and an Investigator’s Global Evaluate (IGA) rating of 3 or 4, indicating realistic or severe disease.
At 16 weeks, an even bigger percentage of patients in the minocycline hydrochloride community done remedy success — an IGA of 0-1 (obvious or near-obvious) with not not up to a two-grade reduction from baseline — as compared with the doxycycline and placebo groups:
- MVOR-1: 65% vs 46% and 31%, respectively
- MVOR-2: 60% vs 31% and 27%
As well, the 2 trials showed vastly bigger suggest reductions in inflammatory lesion counts with minocycline hydrochloride (75-Seventy nine%) versus both doxycycline (60-63%) or placebo (46-47%).
Consistent with the prescribing knowledgedyspepsia become as soon as basically the most fashionable unfavorable match in trials, occurring in 2% of patients versus none assigned to placebo. Given the functionality risks for drug-resistant micro organism, minocycline hydrochloride must finest be frail as indicated.
The drug is contraindicated in patients with a history of hypersensitivity to tetracyclines. Cases of anaphylaxis, serious skin reactions, erythema multiforme, and drug rash with eosinophilia and systemic signs (DRESS) syndrome had been reported in patients the usage of minocycline hydrochloride for zits. Employ in pregnancy and in childhood can lead to permanent teeth discoloration and inhibit bone growth; breastfeeding in the center of command just isn’t instantaneous.
Labeling for the unique plot also entails warnings and precautions about Clostridioides difficile-linked diarrhea, hepatotoxicity, central nervous design results, idiopathic intracranial hypertension, autoimmune syndromes, and metabolic results.
High-tail Scientific acknowledged it expects the drug to be available in the predominant half of of 2025.
-
![author['full_name']](https://clf1.medpagetoday.com/media/images/author/ianIngram_188.jpg)
Ian Ingram is Managing Editor at MedPage This day and helps duvet oncology for the distance.